how many electron does nitrogen have|Electron Configuration Calculator : Manila The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These . Best Cafés in Cabanatuan City, Nueva Ecija Province: Find Tripadvisor traveller reviews of Cabanatuan City Cafés and search by price, location, and more.
how many electron does nitrogen have,Nitrogen has seven protons and seven neutrons in its nucleus, and seven electrons in two shells. It is located in group fifteen, period two and block p of the periodic table. Colourless, gaseous element which belongs to group 15 of the periodic table.
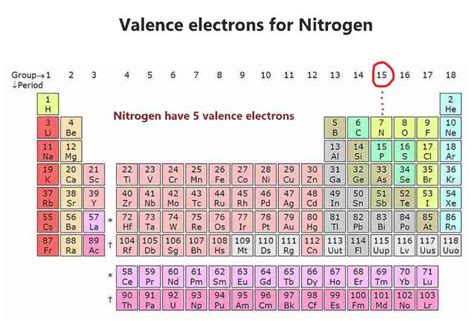
The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These .Ago 5, 2022 — Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total .Hun 30, 2023 — Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 .Hun 8, 2014 — Nitrogen has 5 electrons in its n=2 (outer) shell. There is a quick way of identifying the number of valence electrons - it is the same as the Group number (not for d-block elements, though). Nitrogen is in Group 5, so it has 5 .Electron affinity of Nitrogen is 7 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an .

Electron configuration of Nitrogen is [He] 2s2 2p3. Possible oxidation states are +1,2,3,4,5/-1,2,3 . Many industrially important compounds, such as ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, contain .how many electron does nitrogen haveDis 15, 2019 — If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org .Hun 27, 2024 — For example, the ground state electron configuration of nitrogen ( 1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1s22s22p3) indicates that it has 3 3 3 electrons occupying the 2 p 2 \rm p .
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the .Answer to: An atom of nitrogen has seven protons in its nucleus. How many electrons does it have? By signing up, you'll get thousands of.Electron Configuration CalculatorHun 1, 2024 — How many core electron and valence electron does a nitrogen has? Nitrogen is the element located in group 15, period 2. Thus, its electron configuration is 1s2 2s2 2p3.
Hun 16, 2024 — How many protons neutrons and electrons are in a nitrogen 15? A nitrogen-15 atom has 7 protons, 8 neutrons, and 7 electrons. This is because the atomic number of nitrogen is 7, which corresponds .May 30, 2024 — There are often 7 electrons in nitrogen except when the nitrogen is in its ion form; then it would have 10 electrons. How many valance electrons does the nitrogen atom have? Nitrogen has 5 valence .Mar 4, 2019 — We know that the atomic number of nitrogen is 7.So nitrogen has 7 protons and 7 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1. Step-3:Ago 9, 2021 — 2. You can also look at the electron configuration of Nitrogen to determine the number of valence electrons. The number of electrons in the highest energy level gives you the number of valence electrons. Using this method you will also determine that Nitrogen has 5 valence electrons. The full electron configuration for Nitrogen is 1s 2 2s 2 2p 3.Study with Quizlet and memorize flashcards containing terms like How many valence electrons does each atom of arsenic (As) have? Arsenic is element 33. It is in period 4 and family 15 (5A or the Nitrogen family)., Two representative elements are in the same period of the periodic table. Which statement correctly describes the atoms of the two elements?, Helium is in group 18 of .Ago 12, 2020 — There are two ways to find the number of valence electrons in Nitrogen (N). The first is to use the Periodic Table to figure out how many electrons Nitrogen .Draw an electron-dot structure for acetonitrile, C 2 H 3 N, which contains a carbon–nitrogen triple bond. How many electrons does the nitrogen atom have in its outer shell? How many are bonding, and how many are nonbonding? Exercise \(\PageIndex{10}\)
Each ring has a certain amount of electrons it can hold. The first ring always holds a max of 2 electrons and subsequent rings hold up to 8. Valence electrons are how many electrons are in the outermost ring. For example nitrogen has 7 electrons. 2 go in the first ring leaving 5 in the second ring which are the valence electrons.Mar 23, 2023 — When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Ionic Charges of all Elements (List) . Charge of Nitrogen ion: 3-8: Charge .Hun 16, 2024 — How many electrons does nitrogen have in the 2p orbitals? 3 The electron configuration for nitrogen is 1s22s22p3. How many 2p electrons are in Nitrogen?
Hun 30, 2023 — Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Molecular nitrogen (\(N_2\)) is not reactive at standard temperature and pressure and is a colorless and odorless gas.

Nitrogen-15 presents one of the lowest thermal neutron capture cross sections of all isotopes. Nitrogen-16 is composed of 7 protons, 9 neutrons, and 7 electrons. In nuclear reactors, nitrogen-16 can be used to detect leakages from steam generators. Nitrogen-16 is an isotope of nitrogen generated by neutron activation of oxygen contained in the .how many electron does nitrogen have Electron Configuration CalculatorDis 7, 2016 — The outer electron shell of nitrogen (N) contains 5 electrons.It would need to obtain 3 electrons to fill its shell with 8 electrons in order to have a stable outer electron shell.. An element's capacity to maintain its current state or withstand any changes under usual circumstances is referred to as stability.The fact that it guarantees an element's durability and .An electrically neutral Nitrogen atom has seven electrons. The electronic configuration of Nitrogen is 1s 2, 2s 2, 2p 3. It has two electrons in its inner shell (K) while five electrons are in the outer shell (L). The five electrons are also called valency electrons they .May 21, 2024 — How many electrons would a neutral atom of nitrogen need to lose in order to have a full valence electron shell? A neutral atom of nitrogen (atomic number 7) needs to lose 3 electrons to have a .Hun 18, 2024 — 3 The electron configuration for nitrogen is 1s22s22p3. Why nitrogen is diamagnetic while contains unpaired electrons? If you are going by the electron configuration of nitrogen then the unpaired .
how many electron does nitrogen have|Electron Configuration Calculator
PH0 · Nitrogen (N)
PH1 · Nitrogen
PH2 · Khan Academy
PH3 · How many valence electrons does nitrogen have?
PH4 · Electron Configuration for Nitrogen (N)
PH5 · Electron Configuration Calculator
PH6 · Chemistry of Nitrogen (Z=7)
PH7 · 8.9.2: Chemistry of Nitrogen (Z=7)